
Our
Science
Chronic Kidney Disease
Chronic kidney disease is a public health crisis
Chronic kidney disease (CKD) is a widespread, debilitating, and often fatal disease that affects more than 700 million people worldwide.1 In the US, the number affected is more than 37 million, or one in seven US adults, with healthcare costs exceeding $130 Billion per year.2

As many CKD patients as cancer patients in the US3

As many CKD patients as diabetes patients globally4

Of all new drug approvals
(2017-2021) were in nephrology5
- 1 Cockwell, Paul, and Lori-Ann Fisher, The Lancet 395.10225 (2020): 662-664
- 2 2021 USRDS Annual Data Report: “Epidemiology of kidney disease in the United States”
- 3 American Cancer Society (2019-2021)
- 4 American Society of Nephrology (2018)
- 5 Shayman, James A, JASN 34.3 (2023): 363-365
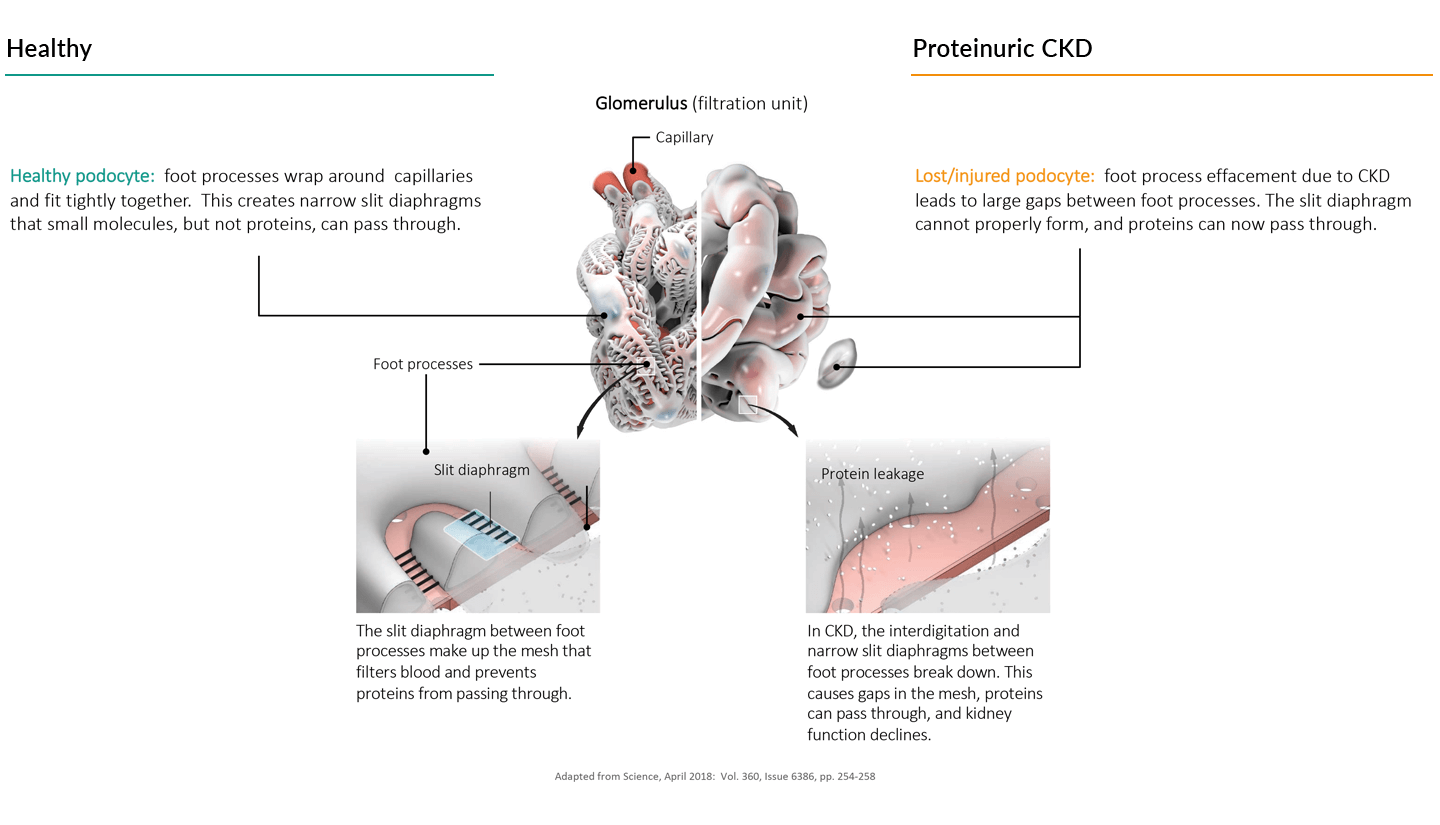
Chronic kidney disease starts with the breakdown of the body’s filtration unit
The kidney is a highly complex organ that carries out several major functions including filtering toxins and excess fluid from blood. CKD occurs when the kidney gradually becomes more damaged and scarred, decreasing this essential function and ultimately resulting in end-stage kidney disease or ESKD. In ESKD dangerous levels of fluids, electrolytes, and waste products accumulate in the body and results in kidney failure. ESKD is fatal without artificial blood filtering (dialysis) or, for those few patients fortunate enough to be matched with a donor, a kidney transplant.
The primary filtering unit of the kidney, the glomerulus, is comprised of a specialized bundle of capillaries with delicate narrow walls through which plasma is pushed under high pressure. The capillaries are surrounded by the glomerular basement membrane or GBM and then unique epithelial cells, called podocytes. Podocytes form processes that interdigitate with processes from neighboring cells. Healthy podocyte processes wrap around capillaries creating narrow channels (the slit diaphragm) through which blood is filtered (see figure below).
Kidney injury occurs when the filtration unit is damaged, often involving podocyte injury, effacement (loss of podocyte processes), or fibrosis. This damage allowing allows proteins to pass into the urine, a, a condition called proteinuria. If this injury lasts for more than a few months, it leads to loss of kidney function and chronic disease, or CKD.
Your kidney filters nearly 200 liters (about 200 quarts) of blood per day. Yet we excrete only about one to one-and-a-half liters per day. It is the job of the rest of the kidney to recover the fluids and nutrients that have escaped into the primary filtrate, as well as actively transport toxins out of the blood and into the urine. In CKD, this secondary filtration system can become overwhelmed trying to recover proteins, as in the case of proteinuric CKD, and this overload of protein reabsorption causes a significant stress on the proximal tubule cells, which leads to tubular injury, the formation of scars, and a further reduction of kidney function.
Two common measures of kidney health are protein levels in the urine, usually expressed as the ratio of albumin (a common blood protein) to creatinine (a muscle toxic waste product) and the estimated glomerular filtration rate (eGFR), which is the amount of fluid filtered from the glomerulus per unit time. A normal albumin-creatinine ratio (ACR) is considered to be less than 30 mg/g. An eGFR above 90 milliliters per minute is in the normal healthy range and eGFR below 60 milliliters per minute for an extended period indicates CKD.
Visit the National Kidney Foundation at www.kidney.org for more information.
Our Focus
There are numerous different disease processes that can damage the glomeruli and proximal tubules and cause proteinuric CKD. The prognoses for these diseases are poor, quality of life is significantly diminished, and there are limited therapeutic options.
At Walden, we focus on developing disease-modifying therapies that directly target the kidney to treat proteinuric CKD and thus improve the lives of patients who not only have declining kidney function but are also faced with higher risks of heart disease, hypertension, and metabolic syndrome.
Learn more about some of the diseases Walden is targeting below:
Focal Segmental Glomerular Sclerosis (FSGS)
Focal Segmental Glomerular Sclerosis (FSGS) is the most common glomerular disease leading to end-stage kidney disease1
FSGS causes scar tissue to form in the filtration units (glomeruli) within the kidney, injuring the podocytes and leading to protein spilling into the urine
On average, FSGS patients will progress to ESKD in ≈10 years; ≈30-50% of the FSGS patients who receive a transplant will have recurrence of FSGS2,3

There are no FDA-approved therapies for FSGS: steroids can slow the disease, but many patients do not respond, and there can be serious side effects4,5

suPAR is a causally implicated in FSGS, and reducing suPAR levels has the potential to stop or slow down the disease6
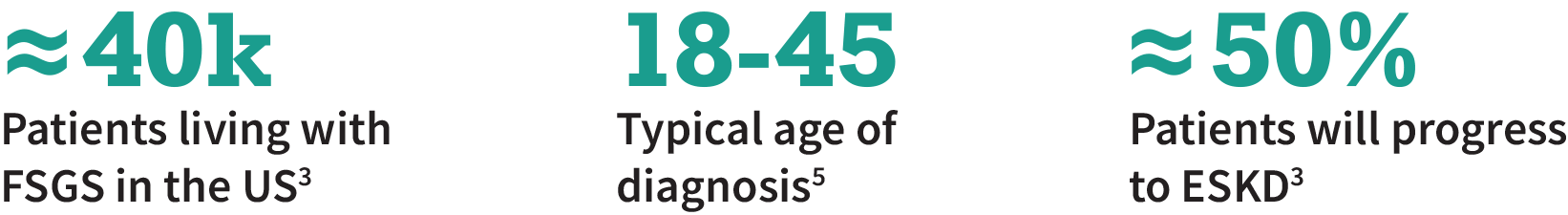
- 1 Shabaka, Amir, Ana Tato Ribera, and Gema Fernández-Juárez, Nephron 144.9 (2020): 413-427
- 2 Canaud, Guillaume, et al., Transplantation Reviews 24.3 (2010): 121-128
- 3 NephCure: Understanding FSGS
- 4 UNC Kidney Center: FSGS
- 5 Sangameswaran, Kothai Divya Guruswamy, Muhammad F. Hashmi, and Krishna M. Baradhi., StatPearls, 2023
- 6 Alachkar, Nada, et al. BMC nephrology 19 (2018): 1-8
Immunoglobulin A Nephropathy (IgAN)
Immunoglobulin A Nephropathy (IgAN) is the most common primary glomerular disease

IgAN is a proteinuric disease caused by buildup of IgA in the kidneys which leads to inflammation and injury of the filtration units (glomeruli)

IgAN typically affects younger adults; ≈30% of patients will progress to ESKD within 20 years1

RAAS blockade is standard of care, but many patients do not respond to treatment1

Despite recent approvals, there is a need for non-steroid and non-immunosuppressive or -non-immunomodulating treatments
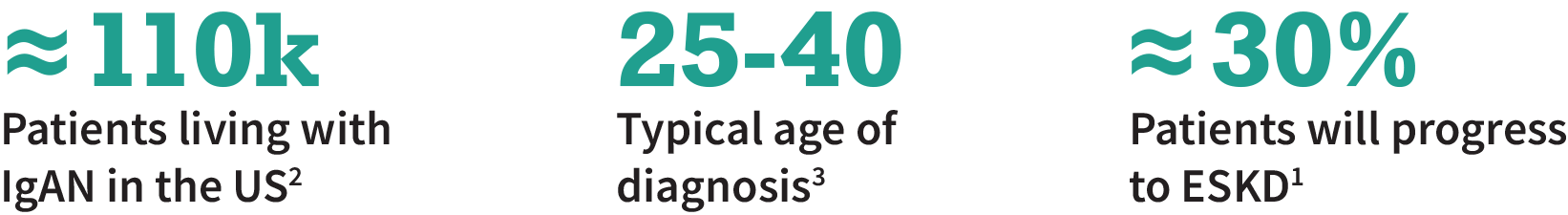
- 1 Cheung C.K., et al., J. Clin. Med. 2021, 10, 2493
- 2 Lerma, Edgar V., et al., Kidney medicine 5.9 (2023): 100693
- 3 Caster, Dawn J., et al. Kidney International Reports 8.9 (2023): 1792-1800
Primary Membranous Nephropathy (MN)
Primary Membranous Nephropathy (MN) is a suboptimally served rare autoimmune disease

MN is most commonly caused by anti-PLA2R antibodies binding to podocytes. This antibody buildup leads to glomerular injury and protein spilling into the urine

The typical time to ESKD for MN patients is ≈10 years; ≈40% of patients who receive a transplant will have recurrence of MN1,2

Current standard of care includes RAAS blockade or immunosuppression, but a significant number of patients do not respond to treatment1-3

There is a need for targeted, fast-acting therapies that avoids the long-term side effects from steroid and immunosuppressive treatment3
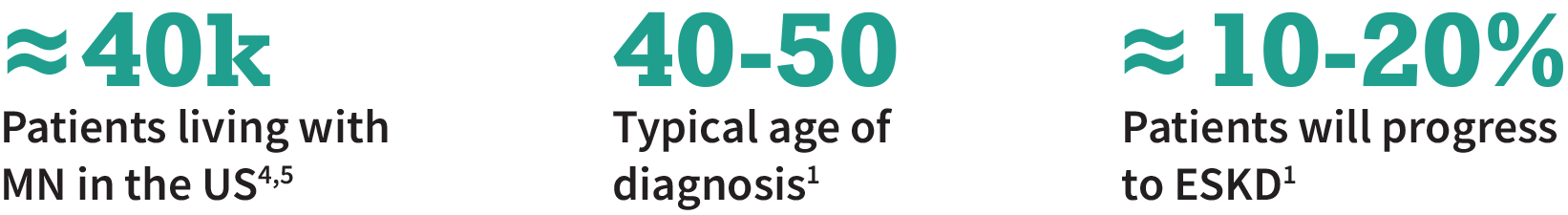
- 1 Couser WG., “Primary Membranous Nephropathy.” Clinical Journal of the American Society of Nephrology: CJASN, 12(6), 2017
- 2 UNC Kidney Center: MN
- 3 KDIGO 2021 clinical practice guideline for the management of glomerular diseases;
- 4 Primary and secondary research, BioMedical Insights, Inc.
- 5 Simon, Christine A., et al., Kidney360 3.6 (2022): 1073-1079
Alport Syndrome (AS)
Alport Syndrome (AS) is a rare, genetic, and progressive kidney disease

AS is a proteinuric disease caused by mutations in the collagen genes. This disrupts the podocytes’ foot processes, causing podocyte loss and kidney injury
Targeting dynamin holds promise to treat AS by crosslinking actin and strengthening the cytoskeleton. This can help stabilize foot processes and prevent their loss1,2

There are no preventive or curative therapies for AS and treatment consists of RAAS blockade or steroids to slow the disease3

There is a significant need for disease-modifying therapies that prevents podocyte loss, e.g., by stabilizing their structure4
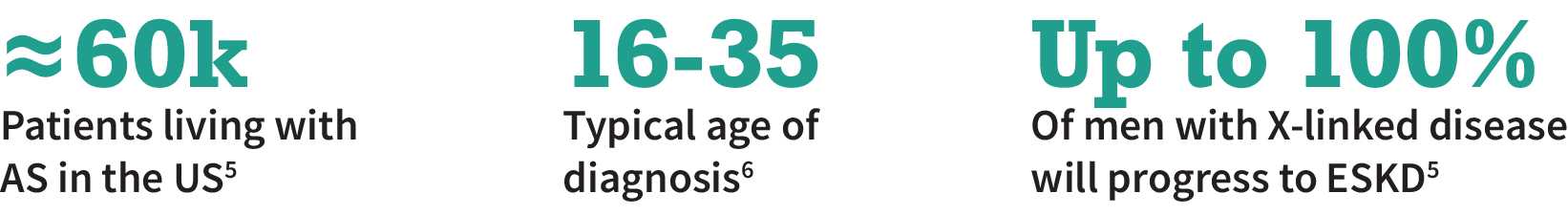
- 1 Mukherjee, Kamalika, et al., Nat Commun 13.1 (2022): 2422
- 2 Ning, Liang, et al., Am J Physiol Renal Physiol 321.1 (2021): F12-F25
- 3 Chavez, Efren, et al., “Novel therapies for Alport syndrome.” Frontiers in medicine 9 (2022): 848389
- 4 Sever, Sanja, and Mario Schiffer, Kidney international 93.6 (2018): 1298-1307
- 5 Warady, Bradley A., et al., Kidney medicine 2.5 (2020): 639-649
- 6 Watson, et al., StatPearls, (https://www.ncbi.nlm.nih.gov/books/NBK470419/)
