
Our
Science
WAL0921
Anti-suPAR antibody targeting suPAR, a pro-inflammatory protein that initiates and aggravates kidney disease.
suPAR is the soluble, circulating form of the urokinase plasminogen activator receptor (uPAR). suPAR is a protein that is present in healthy individuals but elevated in those with CKD. The implication in kidney disease was first suggested in 2008 when Wei, et al. discovered that uPAR is needed to develop proteinuria in mice. Later, in 2011, Wei, et al. showed that suPAR is a circulating factor elevated in FSGS patients, correlates with increased risk of recurrence after transplantation, and causes proteinuria and FSGS-like disease in three different mouse models.
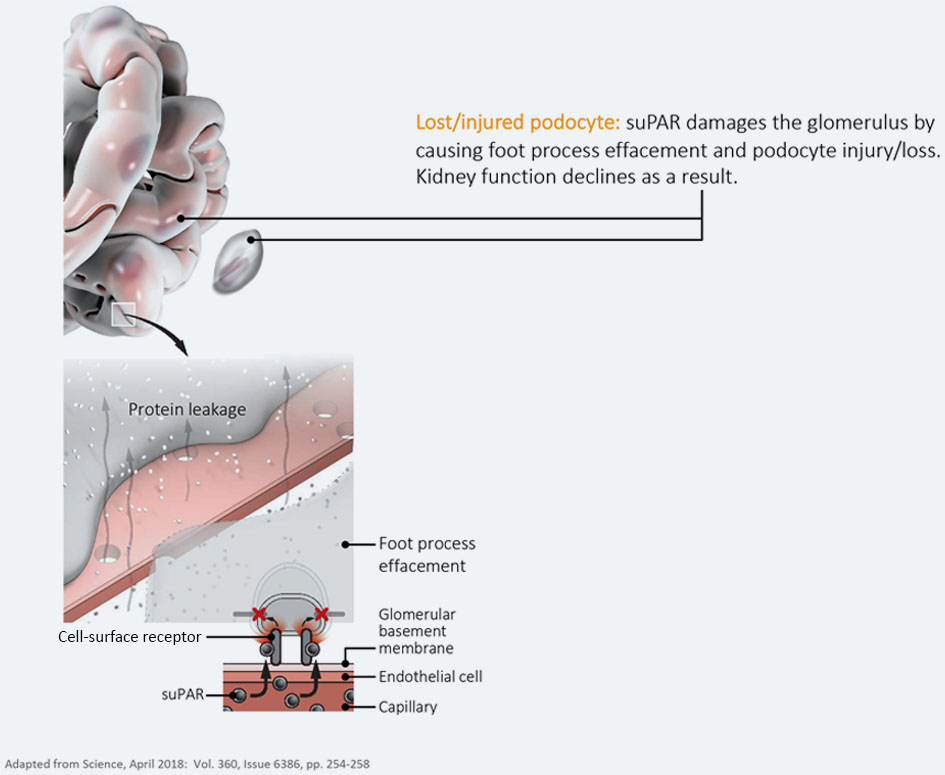
Today, several studies have shown that elevated suPAR levels cause kidney disease progression by activating a signaling cascade which leads to podocyte injury. Preventing this interaction in animals using anti-suPAR antibodies or through a process called plasmapheresis, in individuals with recurrent FSGS, has shown that kidney disease progression can be slowed or halted by the removal of suPAR 6,7,8,9,10.
- 6 Wei, Changli, et al., Nature medicine 14.1 (2008): 55-63
- 7 Wei, Changli, et al., Nature medicine 17.8 (2011): 952-960
- 8 Hindy, et al., JCI, 2022
- 9 Kim, Eun Young, and Dryer, Stuart, Cells 13, (2024) e172
- 10 Hindy, George, et al., J Clin Invest. 132, (2022) e158788
suPAR causes kidney disease
suPAR binds and activates a surface receptor on the podocytes. This changes the podocytes’ shape and causes foot process effacement and injury. As a result, the glomerulus (the kidney’s filter) is disrupted and proteins leak through. This is termed proteinuria and is a hallmark of glomerular kidney disease.

At Walden, we have discovered and developed a humanized monoclonal antibody, WAL0921, that binds suPAR with high affinity and prevents it from activating the signaling that leads to kidney injury.

WAL0921 has completed a Phase 1+ study in healthy subjects. The study showed that a single intravenous dose of WAL0921 was safe, well tolerated, and highly effective at suppressing levels of free suPAR. A Phase 2 study to evaluate safety and activity in patients with kidney disease is underway.
